The Board has issued accessibility standards for medical diagnostic equipment under the “Patient Protection and Affordable Care Act.” The Board also developed guidance on accessible prescription drug container labels. The Board is partnering with the National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx®) Tech accessibility program to host a report on best practices for the design of accessible COVID-19 home tests.
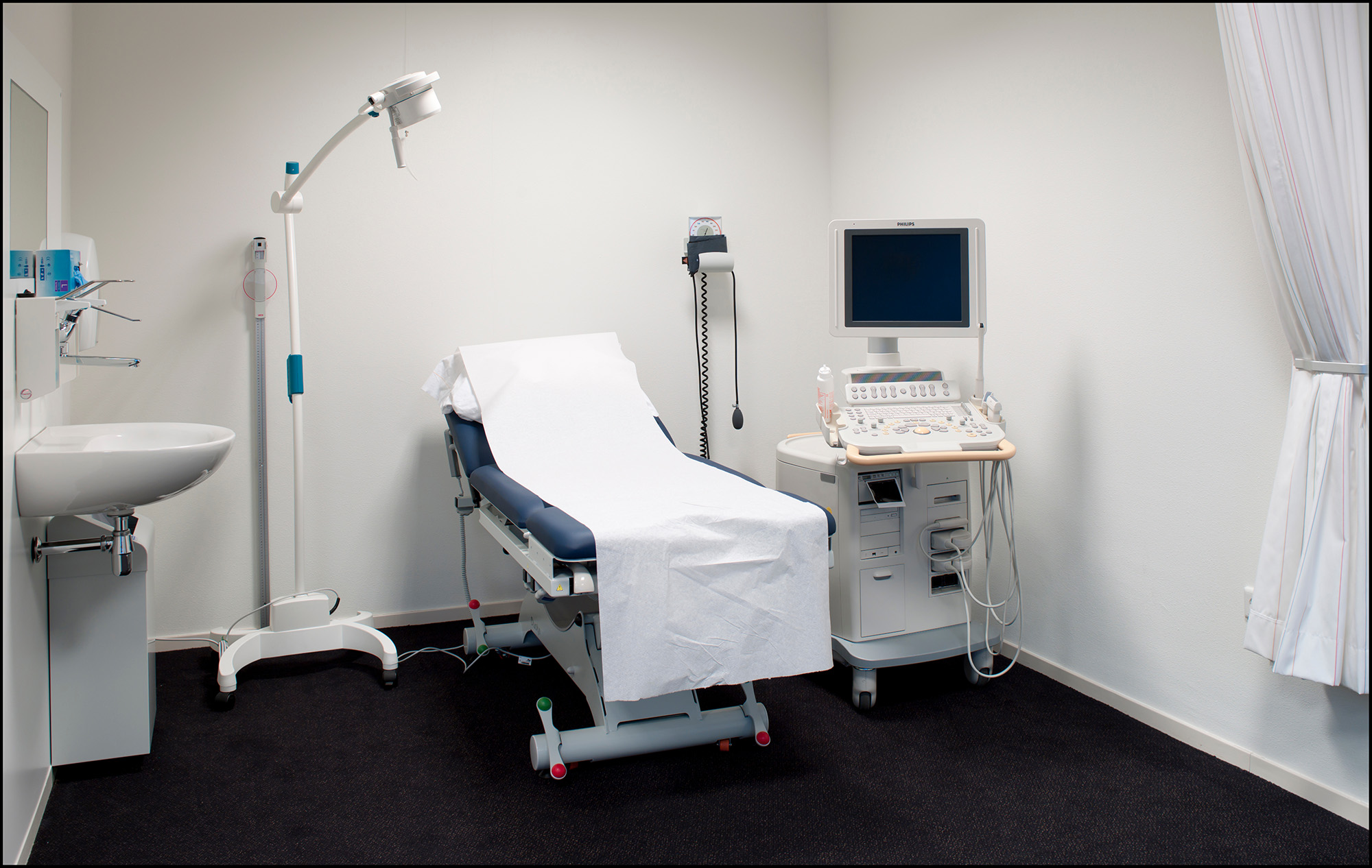
Medical Diagnostic Equipment
These standards address medical diagnostic equipment, including examination tables and chairs, weight scales, radiological equipment, and mammography equipment.
Prescription Drug Container Labels
This advisory non-mandatory guidance was developed by a stakeholder working group to provide best practices on access to prescription drug container labels for people who have vision impairments or who are elderly.
COVID-19 Home Tests
This best practices document captures and publicizes learnings from the NIH RADx® Tech accessibility program to assist manufacturers in the design of COVID-19 and other in vitro diagnostic (IVD) home tests that ensure greater accessibility for users that have no vision or low vision, have a reduced range of dexterity or motor skills, and are aging.

